R&D TOPICS
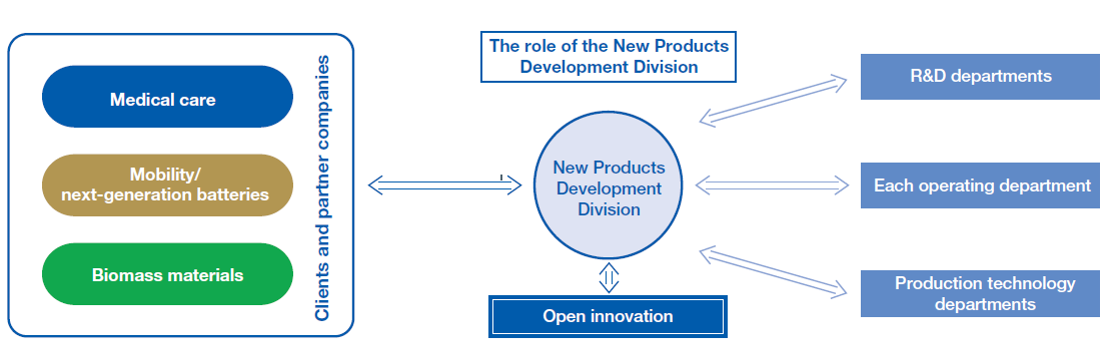
The mission of the New Products Development Division is to create the fourth and fifth pillars of business by identifying the ever-changing social structure and providing solutions to the challenges faced by customers. In particular, we are focusing on three key areas: medical care, mobility, and biomass materials, and are actively promoting the development of new products that take advantage of our philosophy of linking “tangible things,” “intangible things,” and “people,” as well as our core technologies of molecular design, compounding, and analysis.
The Division places the highest priority on contributing to a sustainable society through a thorough understanding of the customer’s perspective. To this end, we pursue groundbreaking innovations in close collaboration among our R&D units, each of our business units, and our production technology departments. We also proactively utilize open innovation from startups and other companies as external resources, aiming to quickly and effectively resolve issues from a broad range of perspectives. E
TOPICS
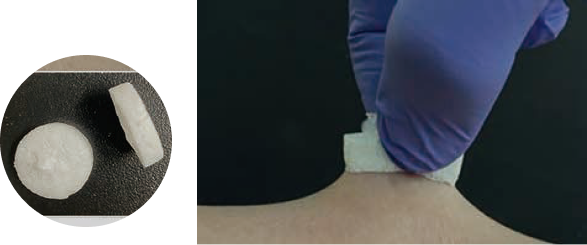
Launched a new product in the medical care division, hemostatic agent Aroncure.
We aim to create original products utilizing our core technologies and commercialize them in the medical care field. As a material that can utilize such polymer technology of our company, we have introduced a new material blended with two types of polymers by a special manufacturing process and started medical device development as "Aron Cure". Aron Cure" is being developed as a unique concept hemostatic agent because of its rapid and strong adhesion to living tissue and its excellent absorption of blood. At the same time, we have been preparing for full-scale entry into the medical device business by establishing a manufacturing and quality assurance system for medical devices.
As the first step in commercialization, the product received medical device approval (in April) under the brand name of "Aron Cure Dental," a wound-covering/protective materials for use in extraction sockets, and is scheduled to go on sale in 2024. The product is expected to shorten hemostatic time due to its excellent adhesion to the living body, and is characterized by uniform quality and high safety. Aroncure" can also be processed into films, sponges, and powders, and is being developed for other medical and cosmetic applications.
Powerful protection for instant wound care
Contributing to medical care issues through the development of new materials Anti-thrombogenic coatings
We focused on anti-thrombogenic coatings as our next target in the medical device field.Medical devices that come in contact with blood, such as ECMO (artificial cardiopulmonary devices), use antithrombotic coatings to prevent the formation of blood clots. Traditionally, heparin, a polymer of animal origin, has been used, but the use of synthetic polymers has increased in recent years due to infection risk and supply stability.Typical examples include non-water-soluble synthetic polymers, which have been put to practical use. However, there are issues such as thrombus generation after prolonged use and limited materials that can be used for coating. We have focused on the "Intermediate Water Theory*" proposed by Professor Ken Tanaka of Kyushu University and developed a coating agent that exhibits superior anti-thrombogenic properties compared to conventional products. Furthermore, we have made improvements to enable physical coating on difficult-to-coat materials used in medical devices, such as polypropylene and silicone, and are promoting practical application in intravitreal and extracorporeal circulatory medical devices.
*Intermediate water theory: Theory of hydration structure of biocompatible polymers.
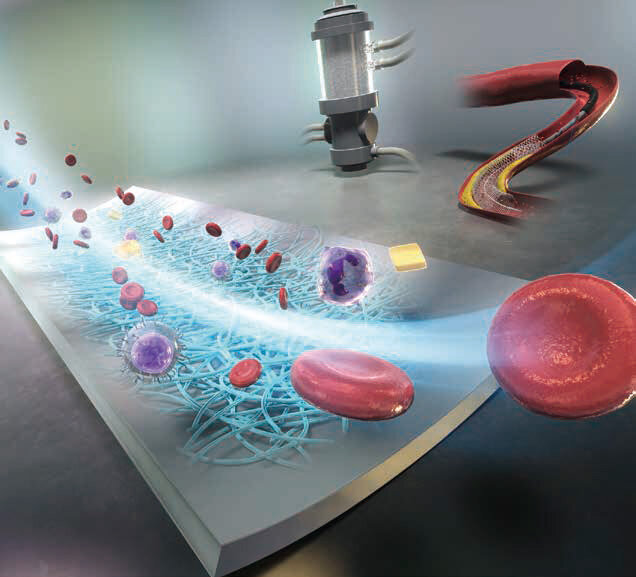
Image of coating agent "Anti-thrombogenic polymer

